|
Conference
|
Abstract Deadline
|
Start Date
|
End Date
|
Location
|
|---|---|---|---|---|
|
CLOSED
|
10.19.2024
|
10.23.2024
|
Denver, Colorado
|
|
|
08.05.2024
|
01.27.2025
|
02.01.2025
|
Aurora, Colorado
|
|
|
TBD
|
TBD - FEB
|
TBD - FEB
|
Honolulu, Hawaii
|
|
|
TBD
|
TBD-FEB
|
TBD-FEB
|
Honolulu, Hawaii
|
|
|
08.08.2024
|
02.26.2025
|
03.01.2025
|
Philadelphia, Pennsylvania
|
|
|
10.11.2024
|
03.25.2025
|
03.29.2025
|
Charlotte, North Carolina
|
|
|
TBD
|
04.24.2025
|
04.27.2025
|
Denver, Colorado
|
|
|
TBD
|
TBD
|
TBD
|
Honolulu, Hawaii
|
|
|
August
|
05.16.2025
|
05.18.2025
|
Minneapolis, Minnesota
|
|
|
Currently Open
|
06.21.2025
|
06.25.2025
|
Kissimmee, Florida
|
|
|
TBD
|
07.28.2025
|
08.01.2025
|
Washington, D.C.
|
|
|
TBD
|
09.12.2025
|
09.13.2025
|
Denver, Colorado
|
Effective July 1, 2016, all new research protocol applications (excludes Cooperative studies) must be submitted through the eProtocol system.
Determine Whether or Not Your Project is Human Subjects Research
To help you determine whether your project is considered human subjects research, download Worksheet 301- Is My Project “Human Subjects Research?”.
Choose your Application Form
To help you determine whether your research qualifies for EXEMPT, EXPEDITED, or FULL-BOARD review, use the Review Category Flowchart.
Below are links to the various application forms you will need:
Complete the application form according to the instructions provided within the form
Submit completed application to the Human Studies Program
Exempt and expedited applications are reviewed in the order that they are received. See the Full-board submission deadlines.
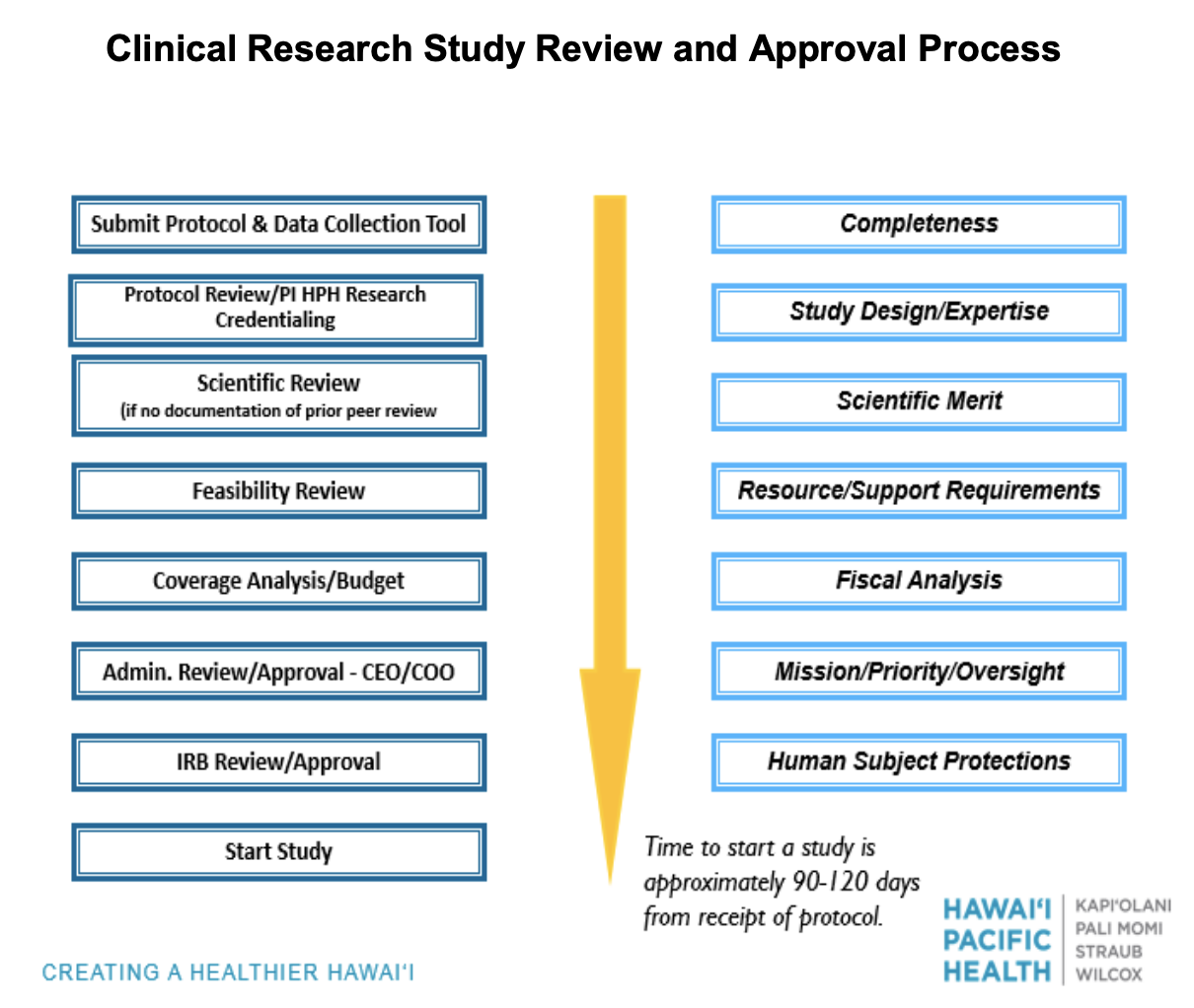
Submit a complete or draft protocol with data collection sheet to HPHRI at research@hawaiipacifichealth.org for a preliminary review of your project. After the preliminary review is complete, a member of the HPHRI team will contact you to complete the required forms for your specific project.
All research conducted at Hawaii Pacific Health must have scientific review by the Scientific Review Committee. HPHRI will submit your project to the Scientific Review Committee on your behalf and contact you with the results of the review.
Should your project require full IRB review, the HPHRI team will assist you with the submission to the Western IRB.
Hawaii Pacific Health uses the Western Institutional Review board as its IRB.
To assure that all clinical researchers understand their responsibility to protect the welfare of their research subjects, Hawaii Pacific Health requires that researchers be “certified” in human subjects protection before conducting research.